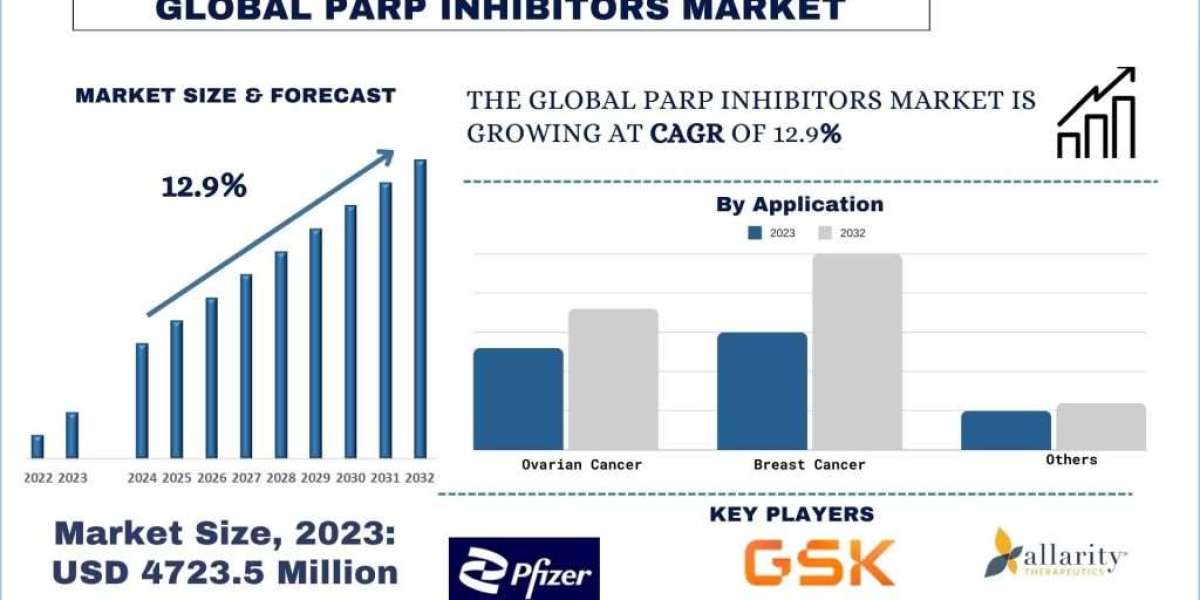
According to a new report by Univdatos Market Insights, PARP Inhibitors Market, is expected to reach USD 13835 Million in 2032 by growing at a CAGR of 12.9%. PARP (Poly (ADP-ribose) polymerase) inhibitors are a class of medications that interfere with the actions of PARP enzymes in cells. These enzymes are involved in DNA repair, particularly in the repair of single-strand DNA breaks. By inhibiting PARP, these medications can prevent cancer cells from repairing their DNA effectively, leading to their death. PARP inhibitors have shown significant promise in the treatment of various cancers, particularly in those with mutations in BRCA genes, such as breast, ovarian, and prostate cancers. They are often used in combination with other cancer treatments, such as chemotherapy or radiation therapy, and have demonstrated efficacy in both early and advanced stages of cancer. However, like all cancer treatments, PARP inhibitors can cause side effects, such as nausea, fatigue, and anemia. Despite these challenges, PARP inhibitors represent a valuable addition to the arsenal of cancer treatments, offering new hope to patients with certain types of cancer.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=62523
The report suggests that the Growing Clinical Pipeline is one of the major factors driving the market in the forthcoming years. The expansion of clinical pipelines is a significant driver in the PARP inhibitor market. The increasing number of PARP inhibitors undergoing clinical trials is a testament to their potential in treating various cancers, particularly those with BRCA mutations. As research progresses, new applications and combination therapies are being explored, enhancing the therapeutic value of PARP inhibitors. This robust pipeline reflects the pharmaceutical industry's commitment to developing innovative cancer treatments, promising improved efficacy and patient outcomes. The continuous influx of new candidates into clinical trials not only broadens the scope of PARP inhibitors but also fuels market growth by attracting investments and fostering advancements in oncology.
Olaparib Segment Gaining Traction in the Market
Olaparib, marketed under the brand name Lynparza, is a pioneering PARP inhibitor developed by AstraZeneca in collaboration with Merck & Co. It has been a breakthrough in the treatment of various cancers, particularly those associated with BRCA1 and BRCA2 mutations. Initially approved by the FDA in 2014 for the treatment of advanced ovarian cancer, Olaparib has since expanded its indications to include breast, pancreatic, and prostate cancers. As one of the first PARP inhibitors to receive regulatory approval, Olaparib has set the benchmark for other PARP inhibitors. Its success has paved the way for further research and development in the field, encouraging other pharmaceutical companies to explore PARP inhibition as a viable cancer treatment strategy. Combining Olaparib with other cancer treatments, such as immunotherapies and targeted therapies, could enhance its efficacy and overcome resistance mechanisms. For instance, in April 2024, US FDA approved a new indication for Olaparib for use in combination with other therapies for treating advanced prostate cancer.
Click here to view the Report Description & TOC https://univdatos.com/report/parp-inhibitors-market/
Conclusion
The PARP inhibitors market has emerged as a pivotal segment within oncology, driven by the growing incidence of cancers, advancements in genetic testing, and the increasing adoption of personalized medicine. These inhibitors have demonstrated significant efficacy, particularly in patients with BRCA mutations and other homologous recombination deficiencies, making them a crucial option in the treatment of ovarian, breast, prostate, and pancreatic cancers. Key players such as AstraZeneca, GlaxoSmithKline, Pfizer, and Clovis Oncology are leading the market, supported by a strong pipeline of new drugs and ongoing research into combination therapies. The favourable regulatory environment and expedited approval processes for breakthrough cancer treatments further bolster market growth. However, challenges such as the high cost of treatment, potential side effects, and the development of resistance highlight the need for continued innovation and strategic approaches to enhance patient outcomes and accessibility. Efforts to identify biomarkers, overcome resistance mechanisms, and develop combination therapies are essential to maintaining the momentum of this market.
Related Report :-
Global Aircraft Landing Gear Market
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website - https://univdatos.com/