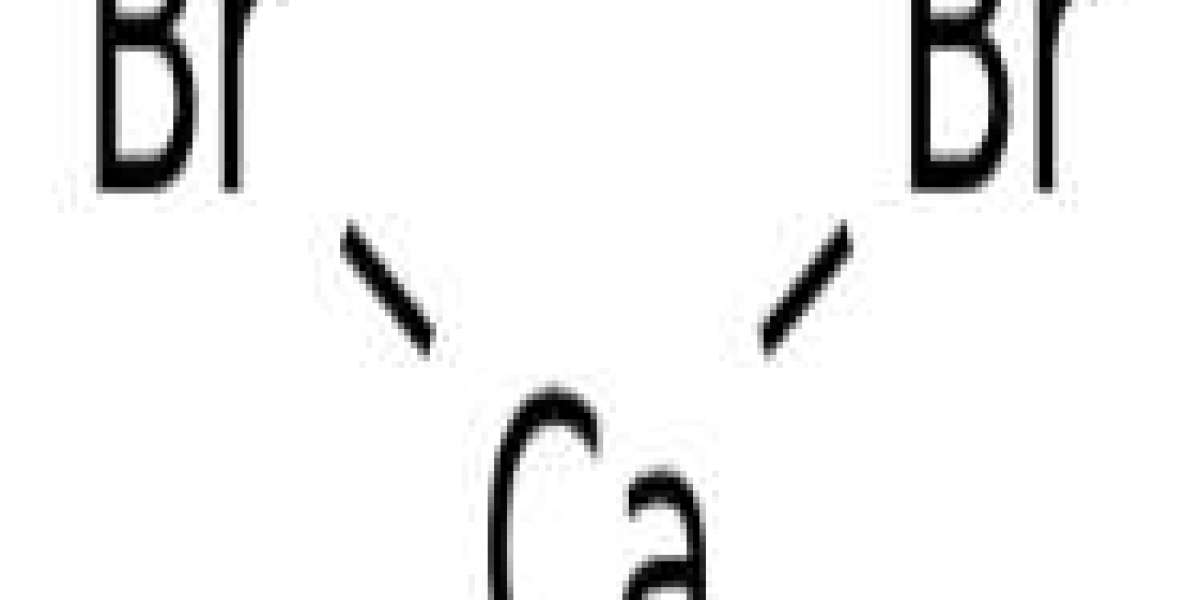Calcium Bromide powder, dissolved in water or other brines, yields completion, workover and packer fluids with a maximum density of 15.3 ppg/1.83 s.g. It is useful in adjusting the density of fresh or recycled workover, completion and packer brines.
Calcium Bromide Solution is used as a completion and workover fluid to control wellbore pressures in upstream oil & gas operations. It can be used alone or mixed with calcium chloride and/or zinc/calcium bromide to achieve a clear, solids-free fluid over a wide range of densities and crystallization temperatures.
Method of producing calcium bromide
An improved method for producing calcium bromide by reacting hydrogen bromide with calcium hydroxide in the presence of water and carbonate ions is disclosed. The improvement comprises maintaining a sufficient pH in the reaction mixture to convert at least a portion of the carbonate ions to a gaseous carbon-oxygen compound thereby removing the carbonate ions from the reaction mixture.
Calcium Bromide Solution is used as a completion and workover fluid to control wellbore pressures in upstream oil & gas operations. It can be used alone or mixed with calcium chloride and/or zinc/calcium bromide to achieve a clear, solids-free fluid over a wide range of densities and crystallization temperatures.
What does Calcium bromide?
Calcium Bromide is a white powder with no odor that is made from the calcium salt of hydro tropic acid, a volatile acid used to make other industrial compounds. Clear brine fluid is it, and also molecular formula is; CaBr2, which has a density of 14.2 lbs/gal . In oil and gas completion and drilling operations, Its combined with calcium chloride to render drill cuttings brine-free.
Usage of Calcium Dibromide
The major use for it is as a component of high-density clear drilling, completion work over, and packing fluids to control well bore pressures. As well as Calcium bromide finds other applications:
In photography
Medicine
Sizing compounds
As a food preservative
In flame retardant blends
What ever Some main chemical properties of calcium bromide include:
Toxicity
Heat of combustion
Ph value
Flammable
Rate of radioactive decay and chemical stability
In addition it’s a white hygroscopic powder available in its anhydrous form .It’s density is 3.35 grams per liter and the melting point is 730 degrees C .it’s edge of boiling over is 1935C . It’s dissolvable in water, ethanol , methanol, and acetone , however it’s insoluble in natural solvents.
Benefits of bromide
In fact It’s non-damaging to the formation
Thermally and chemically stable
It can be blended with other solutions containing bromides and chlorides
And also it’s contains 52% by weight calcium bromide in solution
It can used with calcium chloride brains and dry it to formulate non-damaging fluids of densities from 11.7 lbs/gal to 15.1 lbs/gal