Roots Analysis has announced the addition of ” Novel T-Cell Immunotherapies Market” report to its list of offerings.
The burden of cancer incidence and mortality is increasing rapidly across the world. In 2020, 19.3 million new cancer cases and 10 million deaths due to the disease were reported.[1] In addition, the International Agency for Research on Cancer (IARC) states that, by 2040, the global burden of cancer is expected to grow to 27.5 million new cases and 16.3 million deaths.[2] Although cancer therapeutics are one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. Conventional treatments, such as chemotherapy and radiation therapy, have limited efficacy in late stages of cancers, and are often associated with several side effects. The non-specific mechanism of action also has severe detrimental effects on the patients’ quality of life.[3] Therefore, there is an urgent unmet need for potent and viable therapeutic intervention intended for the treatment of various oncological indications. In this regard, continuous efforts are being made to harness the potential of immune system in controlling and destroying tumors, as well as viral infections.
To request a sample copy / brochure of this report, please visit this - https://www.rootsanalysis.com/reports/novel-t-cell-immunotherapies-market/request-sample.html
The “Novel T-Cell Immunotherapies Market: Focus on γδ T-Cells, Tregs, Activated T-Cells, Virus-driven T-Cells and T-Cell Vaccines - Distribution by Type of T-cell Therapy (Activated T-cell, T-cell Vaccine, Treg and Virus-driven T-cell), Target Indications (Brain Tumor, Pancreatic Cancer, Breast Cancer, Lung Cancer, Nasopharyngeal Cancer, Multiple Myeloma, Hepatocellular Carcinoma, Post-transplant Infection, Diabetes and Others), Key Players and Key Geographies (North America, Europe, Asia Pacific and Rest of the World), Industry Trends and Global Forecast, 2021-2030” report features an extensive study of the current market landscape and the associated future potential. The report highlights efforts of both industry players and academic organizations. Amongst other elements, the report features the following:
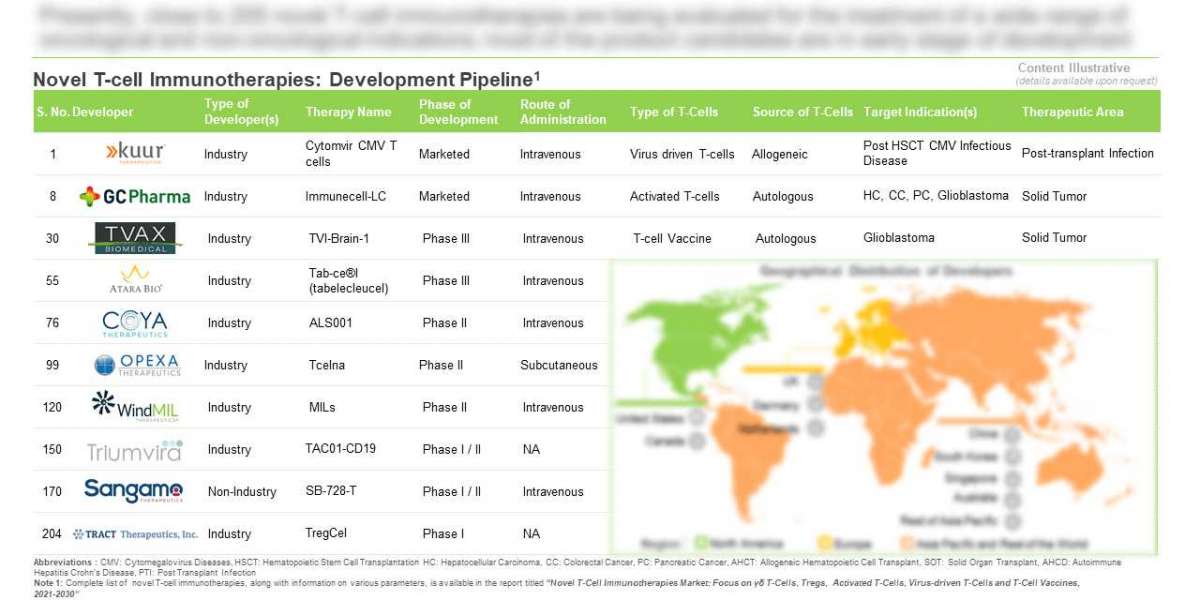
- A detailed assessment of the current market landscape of novel T-cell immunotherapies, with respect to type of T-cell therapy (activated T-cells, virus driven T-cells, Tregs, γδ T-cells, T-cell vaccines and NK T-cells), phase of development (preclinical, phase I, phase I/II, phase II, phase III and marketed), therapeutic area (oncological disorders, infectious diseases, post-transplant infections, autoimmune disorders, metabolic disorders and neurological disorders), popular target indication (lung cancer, breast cancer, cytomegalovirus infection, glioblastoma, hepatocellular carcinoma, acute myeloid leukemia, ovarian cancer, type 1 diabetes mellitus, sarcoma, HIV infections and pancreatic cancer), source of T-cell (autologous and allogeneic), route of administration (intravenous, intratumoral, subcutaneous, inhalation and intramuscular), dose frequency (multiple dose, single dose and split dose), target patient segment (children, adults and seniors), type of therapy (monotherapy and combination therapy) and type of developer (industry and non-industry). In addition, the chapter includes details related to novel T-cell immunotherapies developers, along with information on their respective year of establishment, company size, location of headquarters and most active players (in terms of number of pipeline candidates).
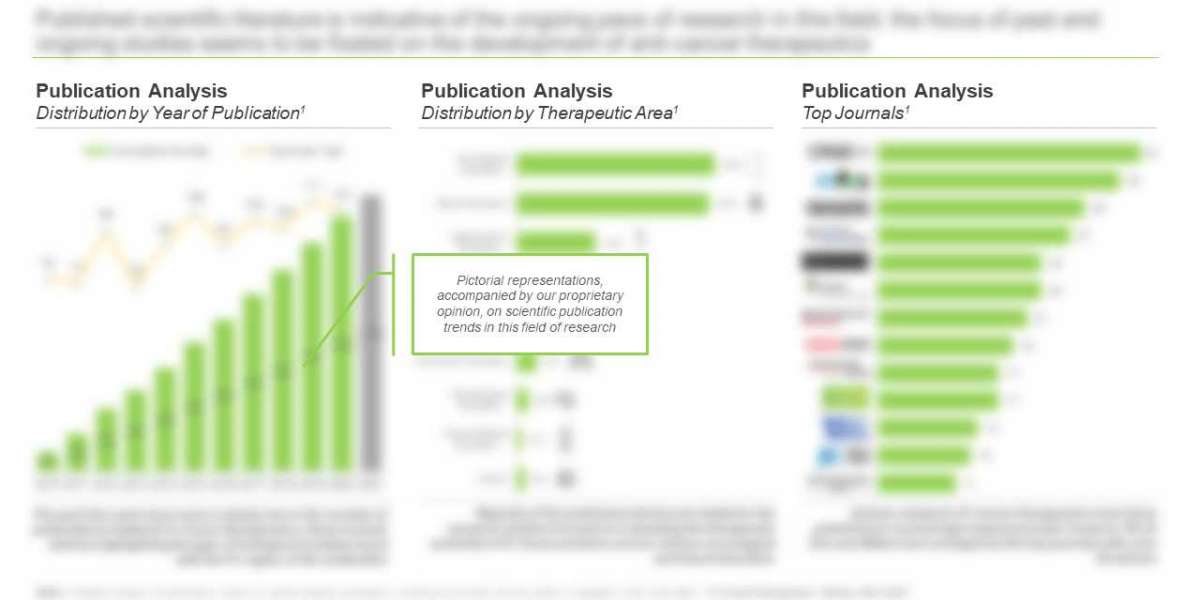
- An in-depth analysis of completed, ongoing and planned clinical studies of various novel T-cell immunotherapies, based on various relevant parameters, such as trial registration year, trial phase, trial recruitment status, study design, type of sponsor / collaborator, leading industry players (in terms of number of registered trials conducted), enrolled patient population and key geographical regions.
- An analysis of the partnerships that have been established in this domain during the period 2015-2021 (till March), covering RD agreements, product development agreements, product licensing agreements, acquisitions, service agreements, manufacturing agreements and other related agreements.
- An analysis of investments that have been made into companies which have proprietary novel T-cell based products / technologies, during period 2014 and 2021 (till March). The various type of funding instances reported in this domain include seed financing, venture capital financing, capital raised from IPOs and subsequent public offerings, grants and debt financing.
- Detailed profiles of clinical stage, novel T-cell therapies (phase I/II or above); each profile features an overview of the therapy, current development status, key clinical trial results, dosage regimen, details related to its manufacturing and recent developments.
- A case study on manufacturing of cell therapy products, highlighting the key challenges associated with the production of such therapies. In addition, it features a detailed list of contract service providers and in-house manufacturers involved in this market.
- Elaborate profiles of prominent players engaged in the development of novel T-cell immunotherapies. Each profile features a brief overview of the company, details related to its financials (if available), product portfolio, recent developments and manufacturing capability.
- A case study on regulatory T-cells, highlighting the mechanism of Tregs, key challenges associated with the production of such therapies and recent developments in the Tregs market.
Activated T-cells (35%) emerged as the most common type of T-cell therapy being developed by stakeholders engaged in this domain; examples include (in alphabetical order) CD8+T cells + Utomilumab (MD Anderson Cancer Center), iNKT cells (Beijing Youan Hospital), SB-728-T (Case Western Reserve University), TAA-T (Children's Research Institute) and TSA-CTL (Beijing Genomics Institute).
This is followed by virus-driven T-cells (28%) and Treg (17%). In addition, numerous research efforts are focused on the development of other novel immunotherapies, such as γδ T-cells, T-cell vaccines and NK T-cells.
For additional details, please visit
https://www.rootsanalysis.com/reports/novel-t-cell-immunotherapies-market.html
You may also be interested in the following titles:
- Regulatory T-Cell Therapies (Tregs) Market, 2021–2035
- CD47 Targeting Therapeutics Market, 2021-2035
- Light Activated Therapies Market, 2021-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/