Characterized by a rapidly growing pipeline and the increasing demand for effective therapeutic interventions, outsourcing of certain manufacturing operations has emerged as a viable option for various microbiome therapeutic developers
Roots Analysis is pleased to announce the publication of its recent study, titled, “Live Biotherapeutic Products and Microbiome Manufacturing Market, 2022–2035”.
The report features an extensive study on the in- house and contract manufacturing organizations within the microbiome industry. The study also includes an elaborate discussion on the future potential of this evolving market. Amongst other elements, the report features:
- A detailed overview of the overall landscape of companies offering contract manufacturing services for the development of microbiome therapeutics.
- A detailed landscape of the live biotherapeutic products and microbiome manufacturing facilities established across key geographical regions (North America, Europe and Asia-Pacific).
- An in-depth company competitiveness analysis of microbiome manufacturing service providers.
- Elaborate profiles of key industry players (large and mid-sized companies, established before 2000) based in North America, Europe and Asia-Pacific that offer contract manufacturing services for microbiome-based live biotherapeutics.
- A list of nearly 70 microbiome-focused drug developers that are likely to partner with manufacturers engaged in this domain.
- A review of the various microbiome-focused initiatives undertaken by big pharma players (shortlisted on the basis of the revenues generated by the top 10 pharmaceutical companies in 2021).
- An analysis of recent developments within the microbiome manufacturing industry, highlighting information on several partnerships and collaborations, mergers and acquisitions, and expansion initiatives that have taken place in this domain, during the period 2016-2021.
- An in-depth analysis of completed, ongoing, and planned clinical studies of various microbiome therapeutics.
- An estimate of the overall, installed capacity for the manufacturing of microbiome-based therapies.
- An informed estimate of the annual clinical and commercial demand for microbiome therapeutics.
- A qualitative analysis, highlighting various factors that need to be taken into consideration by microbiome therapeutics developers while deciding whether to manufacture their respective products in-house or engage the services of a CMO.
- A case study on the current market landscape of microbiome contract research organizations and dietary supplement providers.
- 70 microbiome therapeutics developers are likely to forge strategic alliances with contract manufacturers Around 57% of the drug developers are based in North America, followed by Europe (33%). In addition, 29% of drug developers are dominated by the presence of start-ups (established post 2015), indicating that the market is likely to be driven by such players.
- More than 140 clinical trials evaluating microbiome drugs have been registered worldwide
- Clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 26%, during the period 2016-2021. Of the total, close to 49% of the studies are active and recruiting patients, followed by those that have already been completed (36%).
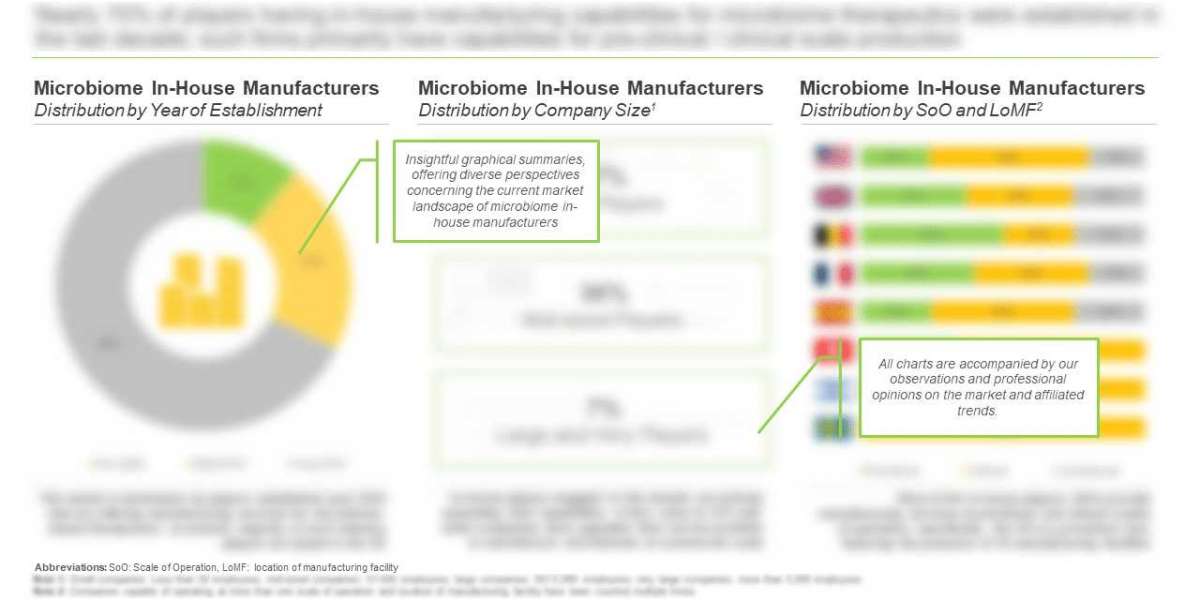
- Approximately 50% of the players carry out microbiome manufacturing operations across all scales of operation
- Nearly 78% of the total industry stakeholders have the required capabilities to manufacture microbiome-based therapies at the commercial scale. In addition, 96% players are capable of manufacturing microbiome-based therapies for clinical purposes.
- A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Type of Formulation
- Solids
- Oral Liquids
- Injectables
- Type of Primary Packaging Used
- Blister Packs
- Glass / Plastic Bottles
- Pouches / Sachets
- Vials
- Scale of Operation
- Clinical
- Commercial
- Company Size
- Small
- Mid-sized
- Large
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
Key companies covered in the report
- Capsugel
- Jeneil Biotech
- UAS Labs
- Biose
- Cerbios-Pharma
- Inpac Probiotics
- NIZO
- Winclove Probiotics
- BJP Laboratories
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/microbiome-contract-manufacturing/306.html
or email [email protected]
You may also be interested in the following titles:
- Peptide Therapeutics: Contract API Manufacturing Market: Industry Trends and Global Forecasts, 2022-2035
- Biopharmaceutical Excipient Manufacturing Market: Industry Trends and Global Forecasts, 2022-2035
- Oligonucleotide Synthesis, Modification and Purification Services Market (2nd Edition): Industry Trends and Global Forecasts, 2021-2031
- Viral Vectors Manufacturing Market, Non-Viral Vectors and Gene Therapy Manufacturing Market
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/