In 2020, revenues from the sales of Avastin®, Roche’s anti-VEGFA antibody based therapeutic, were observed to have declined by 25% due to competition from biosimilars, in the United States, Europe, Japan, and other nations where it is approved
Roots Analysis has announced the addition of the “Avastin® (Bevacizumab) Biosimilars – Pipeline Review and Partnerships” report to its list of offerings.
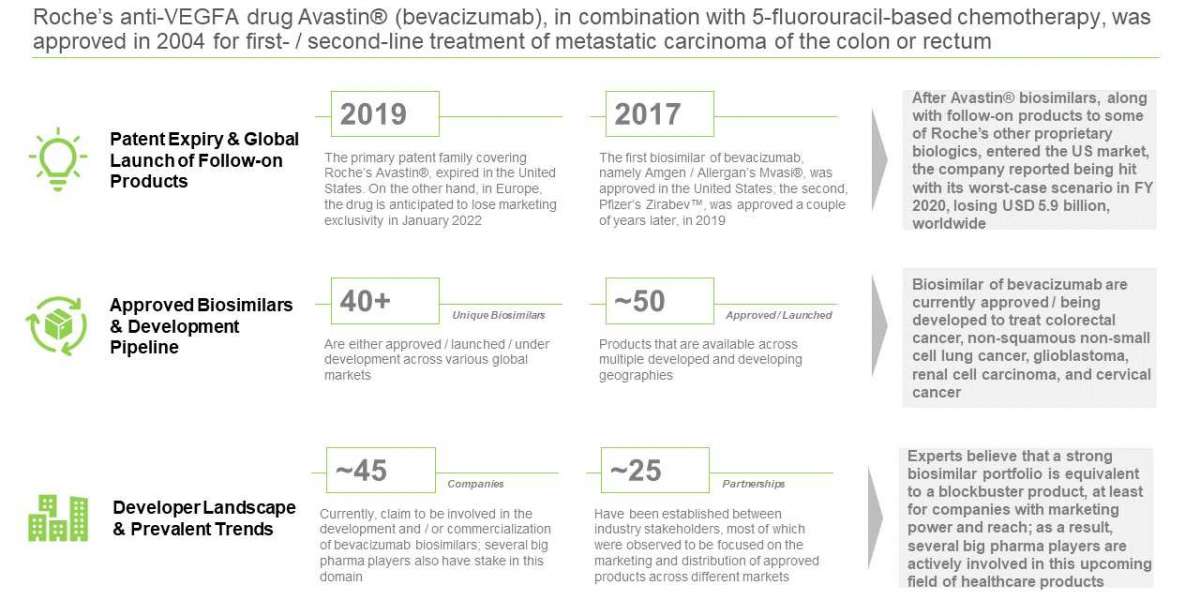
Biosimilars of bevacizumab are currently approved / being developed to treat colorectal cancer, non-squamous non-small cell lung cancer, glioblastoma, renal cell carcinoma, and cervical cancer. Experts believe that a strong biosimilar portfolio is equivalent to a blockbuster product, at least for companies with marketing power and reach; as a result, several big pharma players are actively involved in this upcoming category of healthcare products.
Key Report Highlights
In 2020, Roche lost more than USD 6 billion in international revenues due to biosimilar competition
This substantial erosion in sales is evidence of the market capturing potential of biosimilars, despite the numerous barriers that exist both in the United States and internationally, which prevent such drugs from challenging their much higher-priced originator products
Over 40 companies are currently involved in the development of Avastin® biosimilars
It is worth highlighting that there are 45+ products, representing 9 unique biosimilars of bevacizumab, which have either been approved / launched in various regions of the world. Some of the latest additions to the growing list of bevacizumab biosimilar approvals in the EU, include ALYMSYS® (2021) and AYBINTIO® (2020).
Several biosimilar candidates are presently under development across 130+ trials, in various global regions
Many of such investigational leads are in the late stages of development and for several product candidates, new drug applications (NDAs) / marketing authorization applications (MAAs) have already been submitted to regulators in different nations across the world.
Partnership activity, related to biosimilars of bevacizumab, has grown at a gradual pace since 2009
Most of the deals inked between companies in the same region were product development agreements; on the other hand, the majority of partnerships involving companies across different geographical regions were focused on product distribution.
To request a sample copy / brochure of this report, please visit this
Key Questions Answered
- Who are the key players engaged in the development of biosimilars of bevacizumab?
- In which global marketplaces are Avastin® biosimilars currently available?
- What is the current scenario within the clinical development landscape of bevacizumab biosimilars?
- How many biosimilar development programs have and what were the reasons?
- Who are the key players involved in the commercialization of bevacizumab biosimilars across the world?
- What kind of partnerships have been inked between stakeholders in this domain?
The research includes detailed profiles of companies having approved / launched biosimilars across different global regions; each profile features an overview of the company, information related to its current portfolio of bevacizumab biosimilars, financial information (if available) and key product related specifications.
- Abbott Laboratories
- Alkem Laboratories
- Amgen
- AryoGen Pharmed
- Biocad
- Cadila Pharmaceuticals
- Centus Biotherapeutics
- Dr Reddy’s Laboratories
- Emcure
- Hetero Healthcare
- Innovent Biologics
- Intas Pharmaceuticals
- Laboratorio Elea
- Lupin
- mAbxience
- Pfizer
- Qilu Pharmaceutical
- Reliance Life Sciences
- Samsung Bioepis
- Shanghai Henlius biotech
- Viatris
For additional details, please visit
https://www.rootsanalysis.com/reports/avastin-biosimilars-pipeline-review-and-partnerships.html or email [email protected]
You may also be interested in the following titles:
- HUMIRA® (Adalimumab) Biosimilars – Pipeline Review and Partnerships
- Herceptin® (Trastuzumab) Biosimilars – Pipeline Review and Partnerships
For more information, please click on the following link:
https://www.rootsanalysis.com/reports/avastin-biosimilars-pipeline-review-and-partnerships.html
You may also be interested in the following reports:
- Clinical Trials Software Market
- Precision Medicine Software Developers Market
- Investor Series: Opportunities in the Telehealth Market
- DNA Data Storage Market
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at [email protected]
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/