The exact cause of this rare disorder, which affects 5-17 in 100,000 people, is unknown; however, a complex interplay between different risk factors, including age, genetics and environment, is likely to contribute to the onset of this rare tauopathy
London
Roots Analysis has announced the addition of “Progressive Supranuclear Palsy Therapies Market, 2021-2030” report to its list of offerings.
Presently, there is no cure for PSP and the effectiveness of prescribed generic medications and non-drug therapies to improve the quality of life in patients, is limited. The high unmet patient need and rising caregiver burden has inspired several players to focus their efforts on the development of therapies to control the progression of PSP or manage its symptoms. Consequently, multiple orphan designated drugs, currently in phase II of development, are anticipated to enter the market in the next 3-6 years.
To order this 100+ page report, which features 70+ figures, please visit https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market.html
Key Market Insights
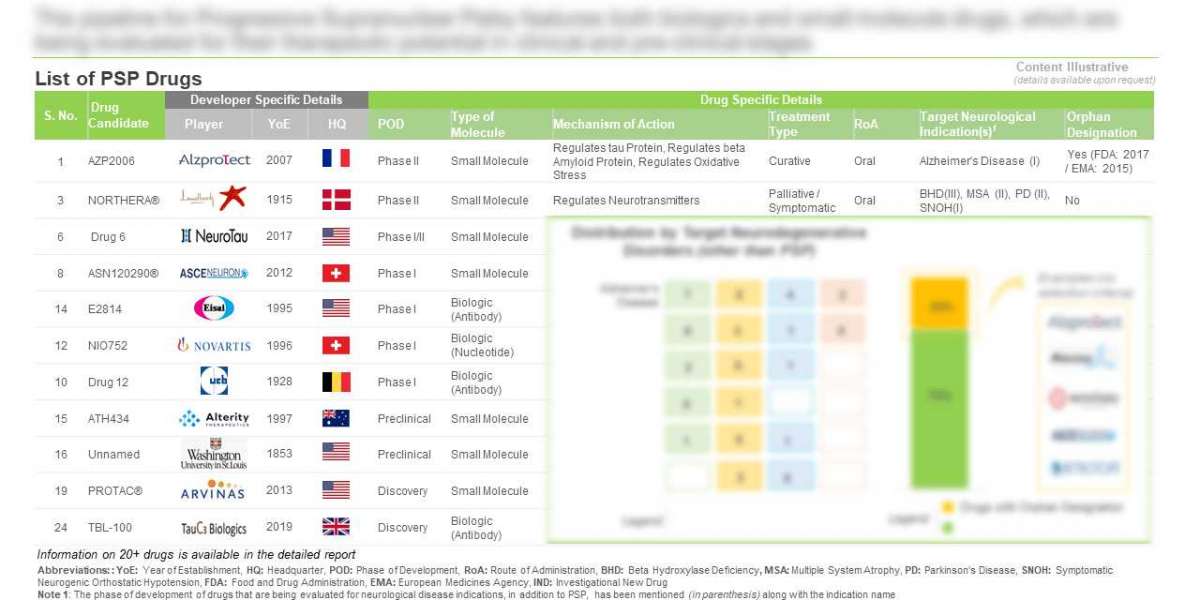
Currently, over 20 drugs are being developed across preclinical and clinical stages
Majority (50%) of the drug candidates are being evaluated in phase I clinical studies to ease the complexities associated with PSP. Further, owing to their ability to cross the blood-brain barrier, small molecule drugs represent ~70% of the PSP pipeline; this is followed by anti-tau antibodies and antisense oligonucleotides. Since majority of the drugs are small molecules, oral route is the most preferred route.
Close to 20 industry and non-industry players are engaged in the development of drugs for PSP
Majority of the industry players (59%) are small firms / start-ups (with less than 50 employees). North America is the current hub, where close to 60% players are based. Further, a few non-industry players, including Washington University and Scripps Research, are leading the development of several early- stage PSP programs.
More than 100 trials, related to PSP, were registered in the last two decades
It is worth mentioning that around 52% of the trials were / are being conducted in Europe and Asia-Pacific region, however, more than 60% of the patients are enrolled in clinical trials conducted in North America. Further, most of the studies are focused on investigating tau targeted radiopharmaceuticals for PSP.
Around 80 eminent scientists, clinicians and industry veterans, with specialization in neurobiology, emerged as key opinion leaders (KOLs), in this domain
Nearly 50% of these KOLs are associated with organizations based in Europe, followed by North America. Multiple individuals from University of California, San Francisco and Toulouse Hospital and University Center are involved in clinical studies for PSP drugs. Further, 76% of these KOLs have doctoral degrees, namely MD and PhD.
Grants worth over USD 300 million have been awarded to support PSP research, since 2010
Till July 2021, over 700 grants have been disbursed to various companies / organizations working in this domain. National Institute on Ageing and National Institute of Neurological Disorders and Stroke emerged as the most active funding institutes, contributing to over 90% of the total grant amount.
In the last two decades, ~2400 articles on PSP, have been published in neuroscience journals
Over the years, there has been a gradual rise in the number of publications related to PSP; nearly 50% of the articles have been published since 2017. With over 150 articles, Parkinsonism Related Disorders and Journal of Movement Disorders emerged as the key journals.
Several partnerships were established by stakeholders in this domain, since 2015
More than 75% of the reported deals were established since 2018, with the maximum activity being reported in 2019. Majority of the instances captured in the report were research and development agreements (33%), followed by licensing agreements (25%).
By 2030, curative therapies, targeting molecular mechanism underlying PSP, are likely to represent over 90% of the market share (in terms of sales revenue)
Growth in this domain is anticipated to be driven by the lack of competition from generics and consequently steering adoption of high-priced curative treatments. It is worth noting that US is likely to capture the highest market share (over 65%). Further, the market in Europe is anticipated to grow at a CAGR of ~25%, in the period 2024-2030.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market.htmll
Key Questions Answered
- What are the prevalent RD trends related to PSP?
- What are the key challenges faced by stakeholders developing PSP therapies?
- Which are the principal therapies being developed for PSP?
- Who are the leading industry and non-industry players in the PSP therapies market?
- Which are the key geographies where research on PSP is being conducted?
- Which are the leading administering institute centers supporting the research related to PSP therapies?
- Who are the key opinion leaders / experts that have contributed to the domain of PSP therapies?
- What kind of partnership models are commonly adopted by stakeholders engaged in this industry?
- What are the factors that are likely to influence the evolution of PSP therapies market?
- How is the current and future market opportunity likely to be distributed across key market segments?
The USD 492 million (by 2030) financial opportunity within the PSP market has been analyzed across the following segments:
- Phase II Drugs
- AZP2006
- RT001
- BRAVYL
- Emeramide
- NORTHERA
- Type of Treatment
- Curative
- Symptomatic / Palliative
- Key Geographical Regions
- North America (US)
- Europe (France, Germany, Italy, Spain, UK)
The report also features detailed transcripts of discussions (in reverse chronological order) held with the following experts:
- Daniel Brennan (Business and Operations Advisor, NeuroTau)
- Fabrizio Stocchi (Director of the Parkinson’s Disease and Movement Disorders Research Center, IRCCS San Raffaele)
The research includes detailed profiles of key players (listed below), each profile features an overview of the company, its financial information (if available), drug portfolio, details on recent developments, as well as an informed future outlook.
- Alzprotect
- Retrotope
- Woolsey Pharmaceuticals
- EmeraMed
- Lundbeck
- NeuroTau
For additional details, please visit
https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market.html
You may also be interested in the following titles:
- Brain Concussion Market, 2021-2031
- Hunter Syndrome: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
- Pediatric Brain Tumors: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
- Neurotrophic Keratitis: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
- Neuromyelitis Optica Spectrum Disorder (2nd Edition): Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
Contact:
Ben Johnson
+1 (415) 800 3415