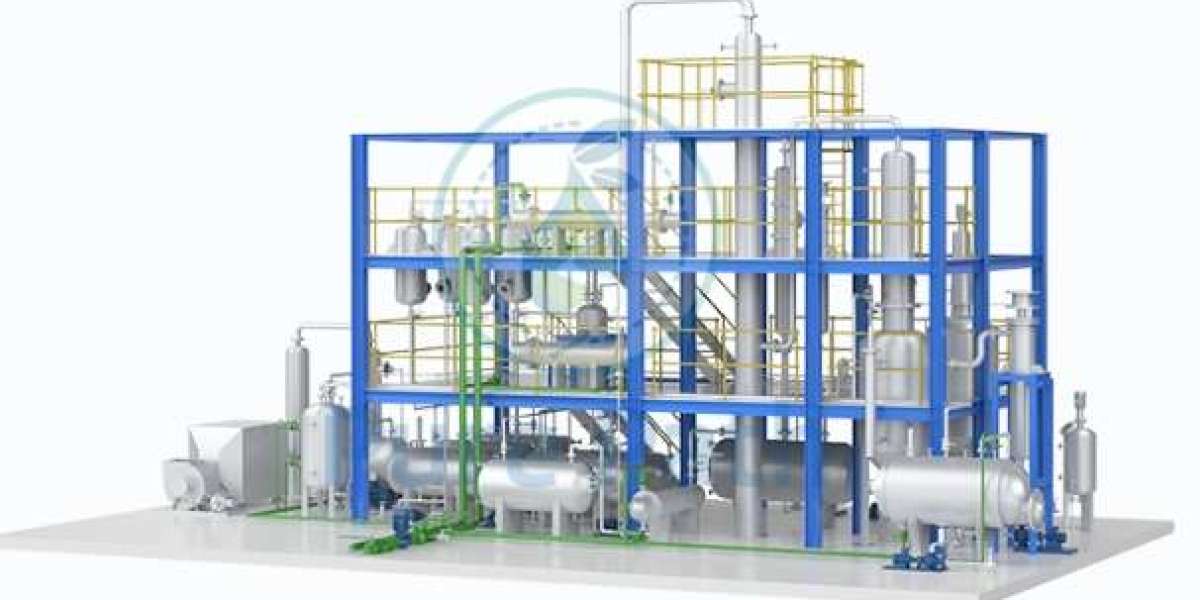
White Spirit Distillation: A Classic Approach
Distillation is a well-established technique for separating components of a mixture based on their boiling points. In the context of white spirit recovery, distillation involves heating the solvent mixture to vaporize the white spirit, which is then condensed and collected.
Advantages of Distillation:
High Purity: Distillation can yield high-purity white spirit.
Versatility: It can be applied to various solvent mixtures.
Established Technology: Mature technology with well-understood principles.
Disadvantages of Distillation:
Energy Intensive: The process requires significant energy input for heating and cooling.
Time-Consuming: Distillation can be a time-consuming process.
Potential for Product Loss: Some solvent may be lost during the process.
Alternative Solvent Recovery Methods
Membrane Separation:
Membrane Filtration: This technique uses semi-permeable membranes to separate solvent from impurities.
Advantages: Low energy consumption, high efficiency, and minimal environmental impact.
Disadvantages: Membrane fouling can reduce efficiency, and it may not be suitable for all solvent mixtures.
Adsorption:
Adsorbents: Solid materials like activated carbon or zeolites can adsorb solvent molecules from a mixture.
Advantages: High selectivity, efficient for low-concentration solvent recovery.
Disadvantages: Adsorbents can become saturated and require regeneration, and the process can be energy-intensive.
Absorption:
Absorbent: A liquid solvent, such as water or a specific organic solvent, can absorb the target solvent from a mixture.
Advantages: High efficiency, especially for gas-phase solvents.
Disadvantages: Requires additional separation steps to recover the target solvent from the absorbent, and it may not be suitable for all solvent mixtures.
Choosing the Right Method
The optimal solvent recovery method depends on several factors, including:
Solvent Properties: The boiling point, polarity, and chemical properties of the solvent.
Contaminant Type and Concentration: The nature and amount of impurities in the solvent mixture.
Desired Purity: The required purity level of the recovered solvent.
Energy Consumption: The overall energy efficiency of the process.
Environmental Impact: The potential environmental impact of the method.
Economic Considerations: The initial investment and operating costs.
In many cases, a combination of methods may be employed to achieve optimal results. For example, distillation can be used to recover the bulk of the solvent, followed by adsorption or membrane separation to remove residual impurities and concentrate the solvent. By carefully considering these factors, industries can select the most suitable solvent recovery method to minimize waste, reduce costs, and promote environmental sustainability.