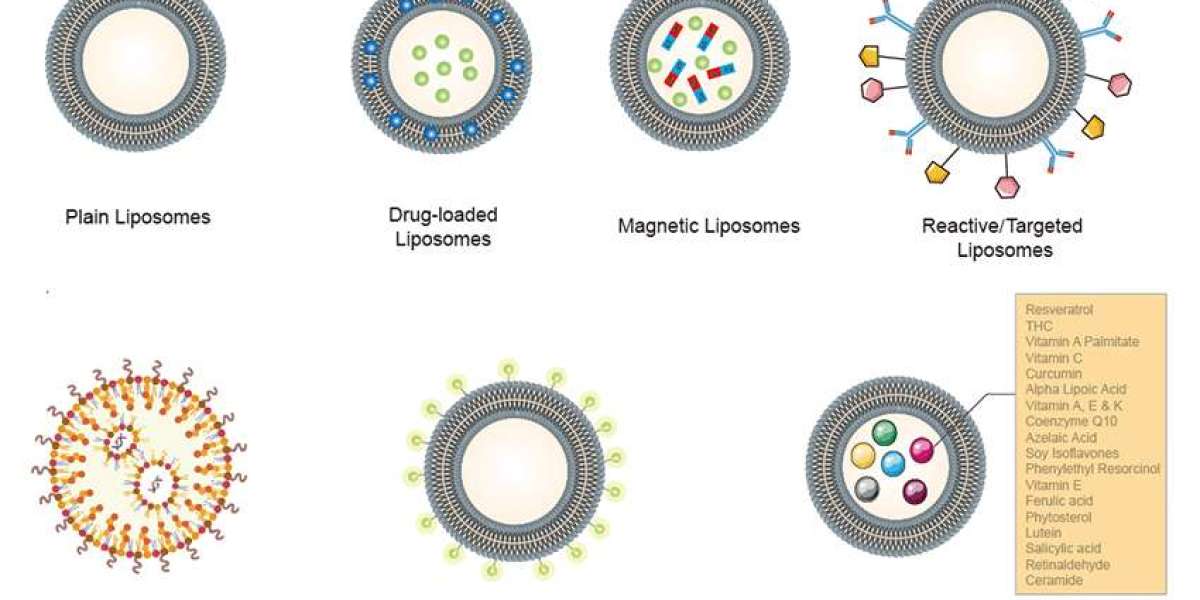
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is proud to announce its expanded capabilities in Customized Liposomes. This advancement allows researchers and developers to leverage the power of liposome technology for a wider range of applications.
Liposomes are small spherical artificial vesicles (typically 50-500 nm in diameter) composed of cholesterol and natural non-toxic phospholipids. Due to their size, hydrophobic and hydrophilic properties (and biocompatibility), liposomes are promising systems for drug delivery. CD Bioparticles now offers customers a comprehensive suite of options for customized liposome design. Its team of experts can tailor liposomes to meet specific needs, including size, lipid composition, and surface charge, ensuring optimal delivery and efficacy. These customized liposomes are ideal for a variety of applications in the pharmaceutical, food, and cosmetic industries.
For example, the 113-O12B-Lipid Nanoparticles Targeted to Lymph Nodes (Catalog: CDCLP24-013-L), the specific lipids synthesized, can allow specific lymph node targeting. This formulation can be used to develop mRNA cancer vaccines without modification of any active targeting ligand. Compared to LNPs formulated with ALC-0315, a key component of the U.S. FDA-approved Comirnaty, 113-O12B showed significantly lower mRNA expression in the liver and higher expression in the lymph nodes after subcutaneous injection.
Another example is FPD-Lipid Nanoparticles (Catalog: CDCLP24-003-L). Fluorinated modification of the 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol)-2000 (PEG-DSPE) (known as FPD) in the LNPs can increase the efficiency of mRNA delivery. A study notes that at relatively low mRNA doses, via intravenous or intraperitoneal injection, FPD increases overall mRNA expression by at least 3-fold, which is not achieved with LNPs prepared with non-fluorinated PEG lipids.
In addition, Sito-Lipid Nanoparticles (Catalog: CDCLP24-007-L) can improve mRNA transport efficiency, and increased PEG concentration in LNPs improves shear resistance and mucus permeability, whereas β-sitosterol gives LNPs a polyhedral shape that facilitates endosomal escape. This formulation is suitable for inhaled mRNA treatment of lung diseases.
CD Bioparticles provides comprehensive solutions to the limitations researchers might encounter in drug delivery and exosome research. Its products address challenges like restricted availability of control liposomes, complex lipid formulation, difficulties incorporating biomolecules onto liposomes, limited tracking methods for cellular interactions, and the overall time-consuming process of liposome development. With customized delivery strategies, precise drug modifications, and advanced platforms, CD Bioparticles can streamline scientific research and development.
With a commitment to innovation and scientific excellence, CD Bioparticles offers researchers comprehensive solutions for their custom liposome needs. The team’s advancements in customized liposomes hold immense promise for targeted drug delivery, personalized medicine, and improved therapeutic outcomes. For more information about these products, please visit https://www.cd-bioparticles.net/customized-liposomes.
About CD Bioparticles
CD Bioparticles is an established drug delivery company that provides customized solutions for developing and manufacturing novel biocompatible drug delivery systems. It specializes in various formulation and drug delivery technologies, from conventional liposomes and PEGylated liposomes to polymer microspheres and nanoparticles for drug delivery. The company also provides contract research services for drug delivery formulation, formulation feasibility study, process development and scale-up, as well as analytical and non-clinical research services.