Poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as a revolutionary class of drugs in the treatment of cancer, majorly ovarian, breast, and prostate cancers. The demand for PARP inhibitors has grown subsequently in past few years, driven by their unique mechanism of action which exploits cancer cells' DNA repair weaknesses. This blog delves into the rising clinical trials and combination therapies in PARP inhibitors, highlighting recent company trials, investment news, and product launches.
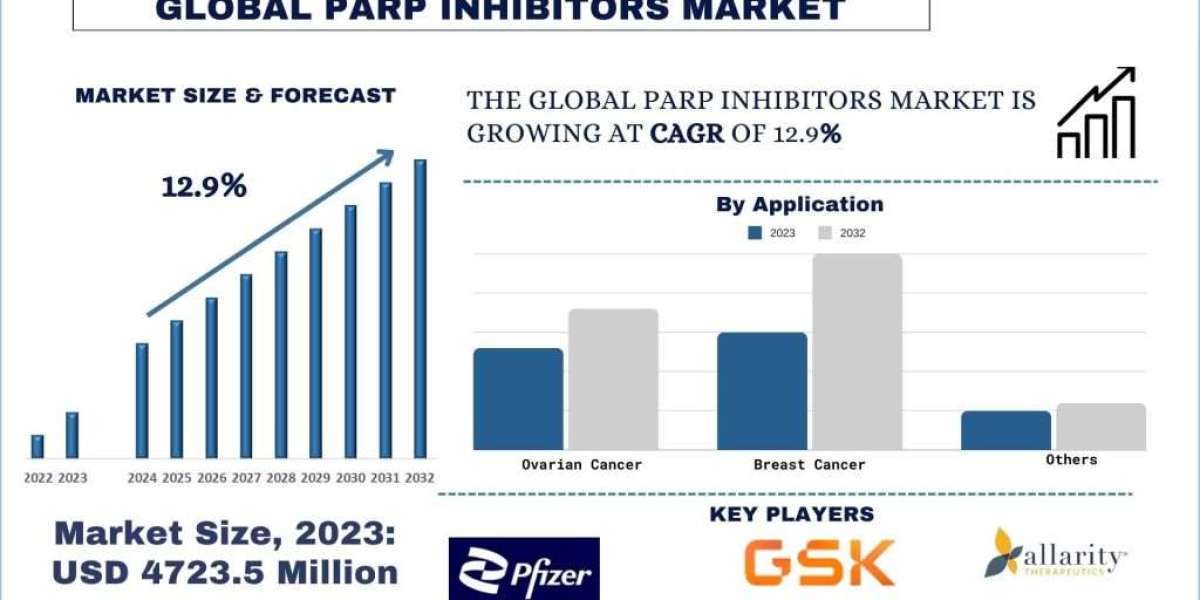
According to the UnivDatos Market Insights analysis, The PARP Inhibitors Market was valued at USD 4723.5 Million and is expected to grow at a strong CAGR of around 12.9% during the forecast period (2024-2032).
For More Detailed Analysis in PDF Format, Visit- https://univdatos.com/get-a-free-sample-form-php/?product_id=62523
The Mechanism of PARP Inhibitors
PARP inhibitors work by inhibiting the PARP enzyme, crucial for repairing single-strand breaks in DNA. When PARP is inhibited, these breaks accumulate, leading to double-strand breaks during DNA replication. Cancer cells, particularly those deficient in other DNA repair mechanisms (like BRCA1/2 mutations), are unable to repair these breaks, leading to cell death. This synthetic lethality makes PARP inhibitors particularly effective in certain cancers.
Rising Clinical Trials
The number of clinical trials investigating PARP inhibitors has surged over the past few years. This rise is fueled by the need for more effective cancer treatments and the potential of PARP inhibitors to be used in combination with other therapies.
Recent Company Trials:
- AstraZeneca and Merck (January 2024):
- Trial: Phase III PAOLA-1
- Combination: Olaparib (Lynparza) with bevacizumab
- Cancer Type: Advanced ovarian cancer
- Outcome: The trial demonstrated a significant improvement in progression-free survival in patients with HRD-positive tumors.
- GlaxoSmithKline (March 2024):
- Trial: Phase II MOONSTONE
- Combination: Niraparib (Zejula) with pembrolizumab
- Cancer Type: Platinum-resistant ovarian cancer
- Outcome: Positive results showing enhanced efficacy compared to monotherapy, supporting further investigation in Phase III trials.
- Clovis Oncology (April 2024):
- Trial: Phase II TRITON2
- Combination: Rucaparib (Rubraca) with enzalutamide
- Cancer Type: Metastatic castration-resistant prostate cancer (mCRPC)
- Outcome: Demonstrated promising activity in patients with BRCA mutations.
Combination Therapies
Combining PARP inhibitors with other cancer treatments such as chemotherapy, immunotherapy, and targeted therapy is a strategy gaining traction. These combinations aim to enhance efficacy, overcome resistance, and improve patient outcomes.
Combination Therapies in Clinical Trials:
- Olaparib with Bevacizumab:
- Used in advanced ovarian cancer, this combination has shown a marked improvement in progression-free survival, particularly in patients with HRD-positive tumors.
- Niraparib with Pembrolizumab:
- This combination is being explored for platinum-resistant ovarian cancer. Preliminary data suggest that it could enhance the immune response against tumors, providing a synergistic effect.
- Rucaparib with Enzalutamide:
- Targeted at mCRPC, this combination leverages the androgen receptor inhibition of enzalutamide and the DNA repair inhibition of rucaparib, showing potential in BRCA-mutant cancers.
Recent Company Strategic News:
The surge in clinical trials and combination therapies has attracted significant investment from pharmaceutical companies and venture capitalists.
Recent Investments:
- Pfizer and Myriad Genetics Collaboration (February 2024):
- Pfizer and Myriad Genetics announced a $500 million investment to co-develop and commercialize a new PARP inhibitor, aimed at expanding the indications and improving delivery mechanisms.
- AstraZeneca’s Expansion (May 2024):
- AstraZeneca committed an additional $300 million towards expanding its PARP inhibitor research, focusing on novel combinations and next-generation inhibitors.
- Clovis Oncology Funding Round (April 2024):
- Clovis Oncology secured $200 million in a funding round led by prominent venture capital firms to advance their pipeline of PARP inhibitor combination trials.
Notable Product Launches in Market
The market for PARP inhibitors is expanding with new product launches aimed at treating various cancers.
Recent Product Launches:
- Lynparza (Olaparib) by AstraZeneca and Merck (January 2024):
- Indication: First-line maintenance treatment for BRCA-mutated advanced ovarian cancer.
- Launch Highlights: Approved based on the positive results of the PAOLA-1 trial, Lynparza combined with bevacizumab offers a new standard of care for patients.
- Zejula (Niraparib) by GlaxoSmithKline (March 2024):
- Indication: Treatment of platinum-resistant ovarian cancer in combination with pembrolizumab.
- Launch Highlights: The combination therapy has shown improved efficacy, leading to accelerated approval.
- Rubraca (Rucaparib) by Clovis Oncology (April 2024):
- Indication: Treatment of BRCA-mutant mCRPC.
- Launch Highlights: The launch was supported by the TRITON2 trial results, offering a new option for prostate cancer patients.
The Future Outlook of PARP Inhibitors
The future of PARP inhibitors looks promising with ongoing research into their use in other cancers, novel combinations, and next-generation inhibitors. The focus is not only on improving efficacy but also on reducing side effects and overcoming resistance.
Ø Expanding Indications
PARP inhibitors are being investigated for their potential in treating a wider range of cancers, including pancreatic and lung cancers. Ongoing trials aim to validate their effectiveness beyond BRCA-mutant cancers, potentially broadening their use.
Ø Overcoming Resistance
One of the challenges in the use of PARP inhibitors is the development of resistance. Research is focused on understanding the mechanisms behind this resistance and developing strategies to overcome it. Combination therapies, such as those with checkpoint inhibitors, are a promising avenue.
Ø Personalized Medicine
Advancements in genomic profiling are paving the way for personalized medicine, where treatments are tailored to the genetic makeup of the individual’s cancer. PARP inhibitors, with their reliance on specific genetic mutations, are at the forefront of this approach.
Explore the Comprehensive Research Overview - https://univdatos.com/report/parp-inhibitors-market
Conclusion
The rise in clinical trials and combination therapies in PARP inhibitors marks a significant advancement in the field of oncology. With substantial investments, successful trials, and new product launches, PARP inhibitors are poised to play a crucial role in the future of cancer treatment. As research continues to expand their applications and overcome current limitations, patients stand to benefit from more effective and personalized cancer therapies.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Website - https://univdatos.com/