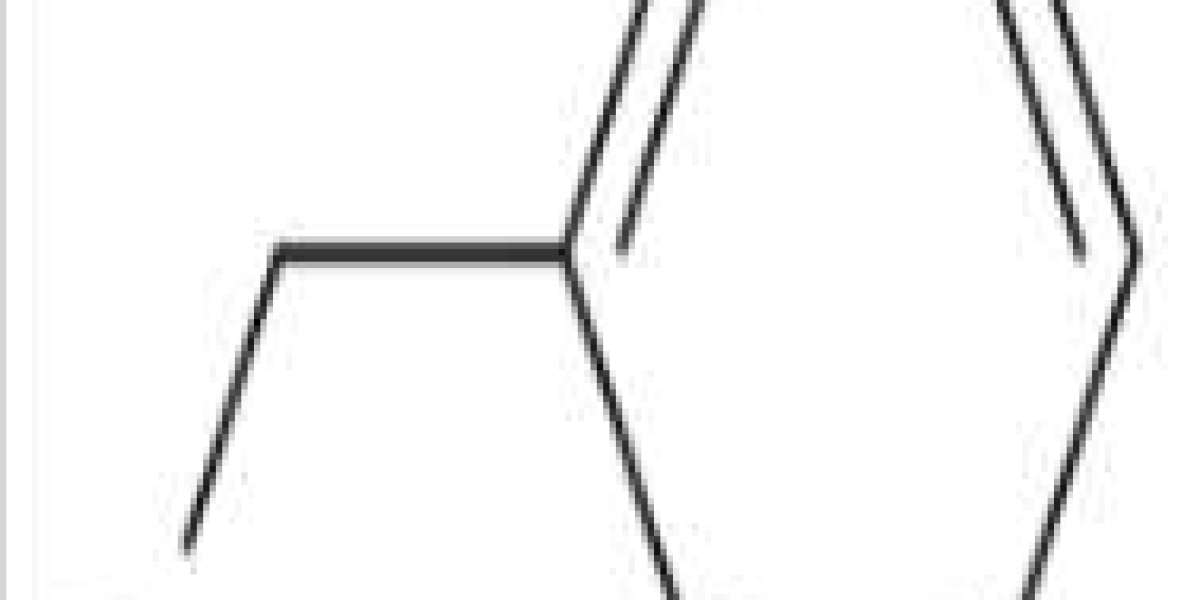
Benzyl bromide is an organobromide compound. It is used in organic synthesis for the introduction of the benzyl protecting group for alcohols and carboxylic acids. Benzyl bromide is a also strong lachrymator, as well as being intensely irritating to skin and mucous membranes. Because of these properties, it has been used as a war gas. Bromine is a halogen element with the symbol Br and atomic number 35. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock.
Benzyl bromide is a member of the class of benzyl bromides that is toluene substituted on the alpha-carbon with bromine. It has a role as a lachrymator.
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
Benzyl bromide appears as a colorless liquid with an agreeable odor. Toxic by inhalation and by skin absorption. It is slightly soluble in water and denser than water (density 1.44 g / cm3 (Aldrich)). A lachrymator. Corrosive to metals and tissue.
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
Benzyl bromide is typically used for installing benzyl protecting groups on alcohols and amines. Benzyl bromide is a strong lachrymator and should be handled in a fume hood. Concentrating reaction mixtures on rotovaps that are not in a fume hood can lead to enough exposure for the irritating effects to be noticed. Purifying crude reaction material on automated purification machines outside a fume hood can also provide sufficient exposure to notice the effects
Search
Popular Posts