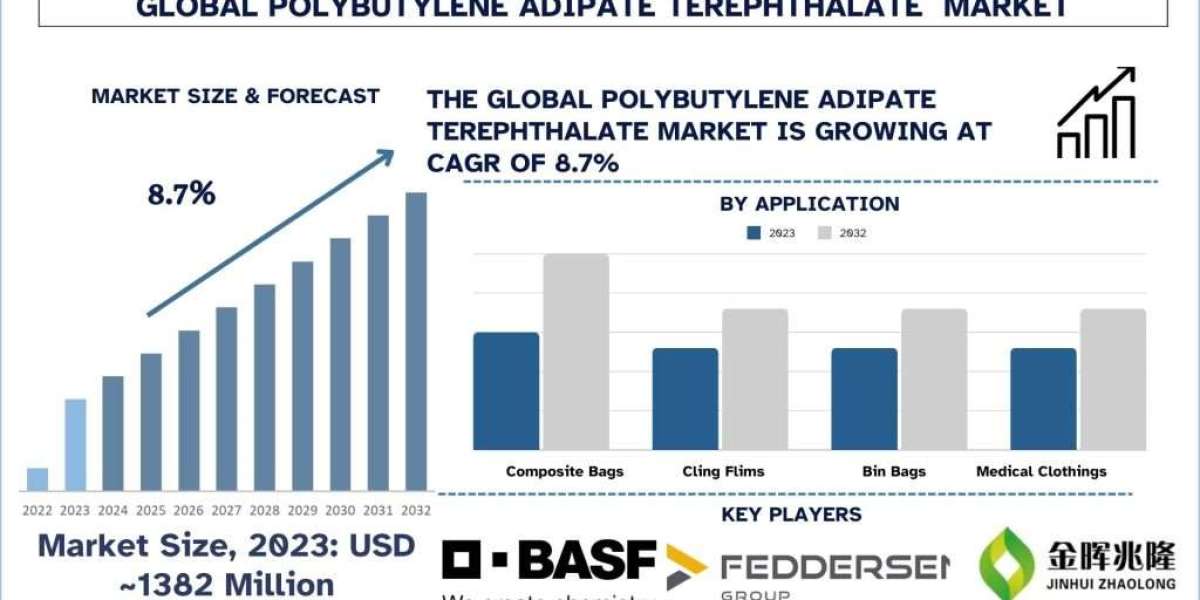
The IV Disinfecting Caps Market globally was valued at USD 1.2 billion in 2022, and it is anticipated to record a rapid revenue CAGR of 7% throughout the forecast period. One of the major factors propelling market revenue growth is the increasing prevalence of Hospital-Acquired Infections (HAIs) and the rising demand for disinfection caps to lower the risk of HAIs. The use of disinfection caps in healthcare facilities is also on the rise. Disinfection caps are attached to injection ports of needle-free connectors to ensure that needle-free connectors remain clean, which reduces the risk of Hospital Acquired Infections (HAIs) such as Central Line Blood Stream Infection (CLABSI).
Healthcare providers are increasingly concerned about HAIs as they lead to higher healthcare expenditures and longer hospital stays. According to the World Health Organization (WHO), hundreds of millions of patients experience HAIs every year, which negatively impact morbidity, mortality, and economic burden. Disinfection caps are becoming popular as a means of lowering the risk of HAIs because they act as a physical barrier, preventing bacteria from entering the catheter hub.
Request a sample Report of IV Disinfecting Caps Market @https://www.reportsanddata.com/download-free-sample/6236
The World Health Organization (WHO) found that over 80% of healthcare-associated infections can be prevented with proper hygiene practices and disinfection equipment. According to research from the Centers for Disease Control (CDC), automated cap washers are more effective than manual scrubbing in removing bacteria from surgical caps and other headwear. A 2017 report by the American Society for Healthcare Engineering suggests that using automated cap washers could reduce contamination rates associated with headwear by up to 50%.
The U.S. Environmental Protection Agency (EPA) states that properly configured disinfection caps can help reduce the spread of infectious diseases and contaminants in healthcare settings. A survey by the Association for Professionals in Infection Control and Epidemiology found that 98% of healthcare facilities use some form of automated cap washer or disinfection caps. In terms of government regulations, the U.S. Food and Drug Administration (FDA) requires all medical devices used for disinfection, including caps and other headwear, to be registered with them before being sold and marketed in the United States. The FDA also sets guidelines for manufacturers regarding labeling, safety testing, and quality control procedures to follow when manufacturing these products. The U.S. Department of Health and Human Services (HHS) has established regulatory requirements for the safe use of disinfection caps in healthcare settings and has set standards regarding labeling and marketing of these products.
Request latest Report Insights of IV Disinfecting Caps Market @https://www.reportsanddata.com/request-latest-insight/6236
Furthermore, revenue growth in the market is being fueled by the increasing adoption of disinfection techniques by healthcare facilities. Healthcare facilities have recently adopted strict disinfection practices to reduce the prevalence of HAIs. Additionally, medical professionals' increasing awareness of the benefits of disinfection caps is also expected to boost market revenue growth. Medical professionals are becoming more aware of the dangers of HAIs and the advantages of using disinfection caps. The availability of disinfection caps in various shapes and sizes to match different catheter and port types is also contributing to their increased use.
Major Companies and Competitive Landscape:
- C.R. Bard, Inc.
- Becton, Dickinson and Company (BD)
- Merit Medical Systems, Inc.
- ICU Medical, Inc.
- B. Braun Melsungen AG
- Nipro Corporation
- DeRoyal Industries, Inc.
- Vygon SA
- Medtronic plc
- Scrubdaddy
Driving Factors in the IV Disinfecting Caps Market are:
- Rising prevalence of Hospital-Acquired Infections (HAIs) and the need to lower the risk of HAIs.
- Increasing use of disinfection caps in healthcare facilities to ensure the cleanliness of needle-free connectors.
- Adoption of strict disinfection practices by healthcare facilities to lower the prevalence of HAIs.
- Growing awareness among medical professionals about the benefits of using disinfection caps to prevent HAIs.
- Availability of disinfection caps in various shapes and sizes to match different catheter and port types.
Restraints in the IV Disinfecting Caps Market are:
- Availability of antimicrobial coated central venous catheters
- High cost associated with disinfection cap
- Stringent regulatory requirements for disinfection cap manufacturing
- Limited awareness and low adoption rate of disinfection cap in developing countries
- Alternative methods of preventing HAIs, such as antibiotic stewardship programs
- Concerns related to the environmental impact of disposable disinfection caps
Request a Report Customization of IV Disinfecting Caps Market @https://www.reportsanddata.com/request-customization-form/6236
Notable Innovations in the IV Disinfecting Caps Market are:
- Development of advanced disinfection cap technology with extended disinfection time and improved effectiveness against a broader range of microorganisms.
- Introduction of disposable disinfection caps that offer a cost-effective solution to reduce the risk of contamination.
- Integration of smart technology with disinfection caps, such as RFID (Radio-frequency identification), to track usage and ensure compliance with disinfection protocols.
- Use of materials that are more eco-friendly and biodegradable in disinfection cap production.
- Development of disinfection caps that are compatible with a wide range of catheter types and sizes to offer flexibility in use.
About Reports and Data
Reports and Data is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyze consumer behavior shifts across demographics, across industries, and help clients to make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, TouchPoints, Chemicals, Products, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Reports and Data has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provide our clients with the ability to secure an edge over their respective competitors.
Contact Us:
John W
Head of Business Development
Reports And Data | Web: www.reportsanddata.com
Direct Line: +1-212-710-1370
E-mail: [email protected]
LinkedIn | Twitter | Blogs
Read the innovative blog at https://www.reportsanddata.com/blogs
https://www.droit-inc.com/banner_click.php?id=102url=https://www.reportsanddata.com
https://auth.mindmixer.com/GetAuthCookie?returnUrl=https://www.reportsanddata.com
https://secure2.atomiclearning.com/platform/?referer=https://www.reportsanddata.com
https://www.db.lv/ext/https://www.reportsanddata.com
https://promosimple.com/api/1.0/routedevice?durl=reportsanddata.com