To order this 150+ page report, which features 100+ figures and 5+ tables, please visit
https://www.rootsanalysis.com/reports/gene-editing-market.html
Key Inclusions
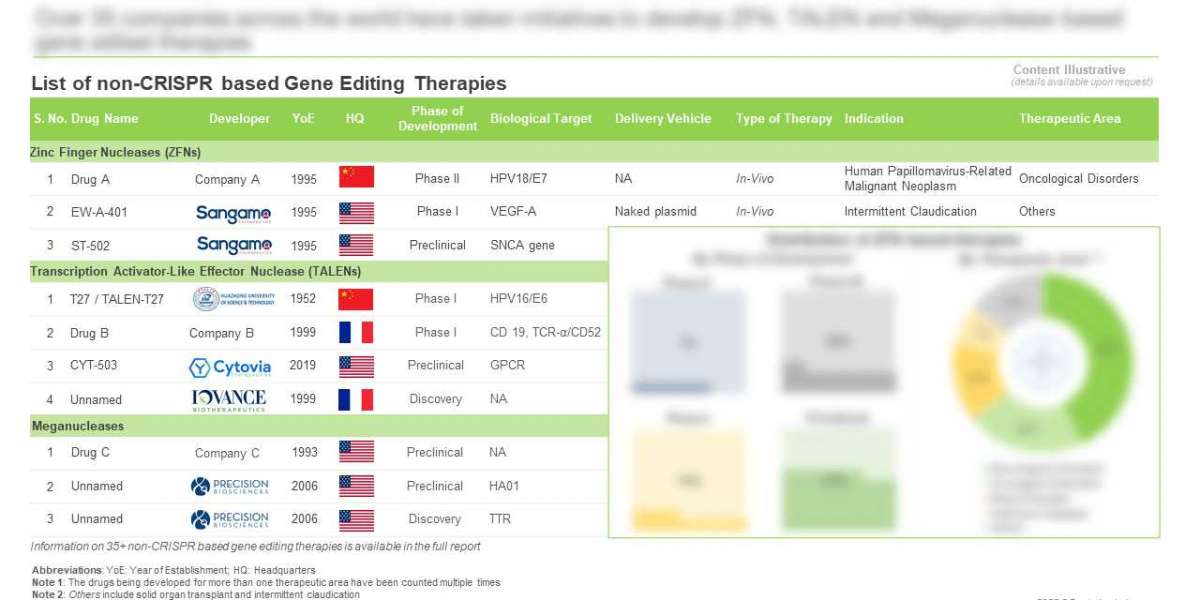
- A detailed assessment of the current market landscape of ZFNs based therapies, based on several relevant parameters, such as phase of development (clinical and preclinical), biological target(s) (BCL 11A, C9ORF72, CD 19, HLA-A2, HTT gene, MAPT, SNCA gene, VEGF-A, HPV16/E7 and HPV18/E7), delivery vehicle (non-viral and naked plasmid), target indication(s), therapeutic area(s) (Blood Disorders, Infectious Diseases, Neurological Disorders, Oncological Disorders and Others) and type of therapy (in-vivo and ex-vivo). In addition, it provides details on the companies engaged in the development of ZFNs based therapies, along with information on their year of establishment, company size and location of headquarters.
- A detailed assessment of the current market landscape of TALENs based therapies, based on several relevant parameters, such as phase of development (clinical and preclinical), biological target(s) (iNK, CD19, BCMA, TCR-α/CD52, CD22, CD38, CS1, GPCR, HPV18/E7, HPV16/E6, HPV16/E7 and HPV18/E6), target indication(s), type of cells (T Cells, NK Cells, HPV positive and B Cells), type of therapy (in-vivo and ex-vivo), cell / gene therapy (NK-Cell Therapy, T-Cell Therapy and CAR-T), gene editing approach (insertion and deletion) and route of administration (intravenous, topical and intravaginal). In addition, it provides details on the companies engaged in the development of TALENs based therapies, along with information on their year of establishment, company size and location of headquarters.
- A detailed assessment of the current market landscape of Meganucleases based therapies, based on several relevant parameters, such as phase of development (clinical and preclinical), biological target(s) (HA01, TTR, PCSK9 and ApoC3), delivery vehicle (AAV9 and lentiviral), target indication(s), therapeutic area(s) (Genetic Disorders, Liver Disorders, Kidney Disorders, Rare Disorders, Ocular Disorders and Oncological Disorders), type of cells, type of therapy, and route of administration. In addition, it provides details on the companies engaged in the development of Meganucleases based therapies, along with information on their year of establishment, company size and location of headquarters.
- Elaborate profiles of key players engaged in the development of ZFNs, TALENs and Meganucleases based therapies. Each profile features a brief overview of the company, details on its drug candidates, its financial information (if available), recent developments and an informed future outlook.
- A detailed analysis of completed and ongoing clinical trials of various ZFNs, TALENs and Meganucleases based therapies, based on different parameters, such as trial status, trial registration year, type of sponsor / collaborator, study design, number of patients enrolled. In addition, the chapter highlights year-wise trend of completed and recruiting trials, age category of the patients enrolled, active industry and non-industry players and location of the trials.
- A detailed review of more than 10,000 peer-reviewed, scientific articles related to research on ZFNs, TALENs and Meganucleases based therapies, based on parameters, such as year of publication, emerging focus area, type of publication, therapeutic area, and target indication. The chapter also highlights the top journals and top authors (in terms of number of articles published).
- An in-depth analysis of academic grants that have been awarded to various research institutes for projects related to ZFNs, TALENs and Meganucleases based therapies, during the period, 2017-2021, based on several parameters, such as year of grant award, amount awarded, type of funding institute center, popular NIH departments, support period, emerging focus area, purpose of grants, grant activity code, local recipients, type of recipient organization study section and type of grant application. Further, the chapter also highlights the popular recipient organizations, (in terms of number of grants and amount awarded) and prominent program officers.
- An analysis of the partnerships that have been established in this domain till 2021, covering instances of research and development agreement, clinical trials agreement, merger / acquisition, product development and commercialization agreement, licensing agreement, asset acquisition and product development and manufacturing agreement.
- A detailed analysis of the various investments made till 2021, including grants, seed financing, venture capital financing, IPOs, secondary offering, other equity, post IPO equity and equity crowdfunding in the companies focused on the development of myeloid cells targeting therapeutics.
- An in-depth analysis of the patents that have been filed / granted for ZFNs, TALENs and Meganucleases till 2021. It highlights trends across the key parameters associated with the patents, including type of patent, issuing authority / patent offices involved, Cooperative Patent Classification (CPC) symbols, emerging areas (in terms of number of patents filed / granted), type of company, leading industry and non-industry players (on the basis of number of patents) and individual patent assignees (in terms of size of intellectual property portfolio).
- A list of key opinion leaders (KOLs) within this domain, and their assessment (based on the strength and activeness) represented in the form of 2×2 matrices. The chapter also includes an analysis evaluating the (relative) level of expertise of different KOLs, based on number of publications, number of citations, participation in clinical trials, number of affiliations and strength of professional network.
- A case study on the CRISPR based therapeutics that are currently in different stages of development. It features a detailed analysis of pipeline molecules, based on several relevant parameters, such as target therapeutic area (autoimmune disorders, cardiovascular disorders, dermatological disorders, genetic disorders, hematological disorders, immunological disorders, infectious diseases, inflammatory disorders, metabolic disorders, muscular diseases, neurological disorders, oncological disorders, ophthalmic diseases and others), phase of development (discovery, preclinical and clinical), approach of therapy (ex vivo and in vivo), cell source (autologous and allogeneic), type of therapy (CAR-T therapy, HSC therapy, T cell therapy, Phage therapy and others), and the type of technology used.
- A detailed market forecast, featuring analysis of the current and projected opportunity across the following key geographical regions, including:
- North America
- Europe
- Asia Pacific
To request sample pages, please visit
https://www.rootsanalysis.com/reports/gene-editing-market/free-insights.html
Key Questions Answered
- Who are the players engaged in the development of gene editing therapies beyond CRISPR?
- Which are the key drugs being developed across early and late stages of development?
- Which companies are actively involved in conducting clinical trials for ZFNs, TALENs and Meganucleases based therapies?
- What is the focus area of various publications related to ZFNs, TALENs and Meganucleases based therapies?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What is the trend of capital investments in the gene editing beyond CRISPR market?
- How has the intellectual property landscape related to ZFNs, TALENs and Meganucleases based therapies evolved over the years?
- How is the current and future opportunity, related to ZFNs, TALENs and Meganucleases based therapies, likely to be distributed across key market segments?
You may also be interested in the following titles:
- CRISPR based Therapeutic Market by Type of Therapy, 2021-2030
- Gene Editing Services Market- Focus on CRISPR, 2019-2030
Contact:
Ben Johnson
+1 (415) 800 3415